From 24 to 28 November 2025, in Lyon, France, at the WHO Academy, a meeting was held between the national regulatory authorities of countries that are members of the Global Network of National Quality Control Laboratories for Pharmaceuticals (WHO-GNP) and the National Control Laboratory Network for Biologicals (WHO-NNB).
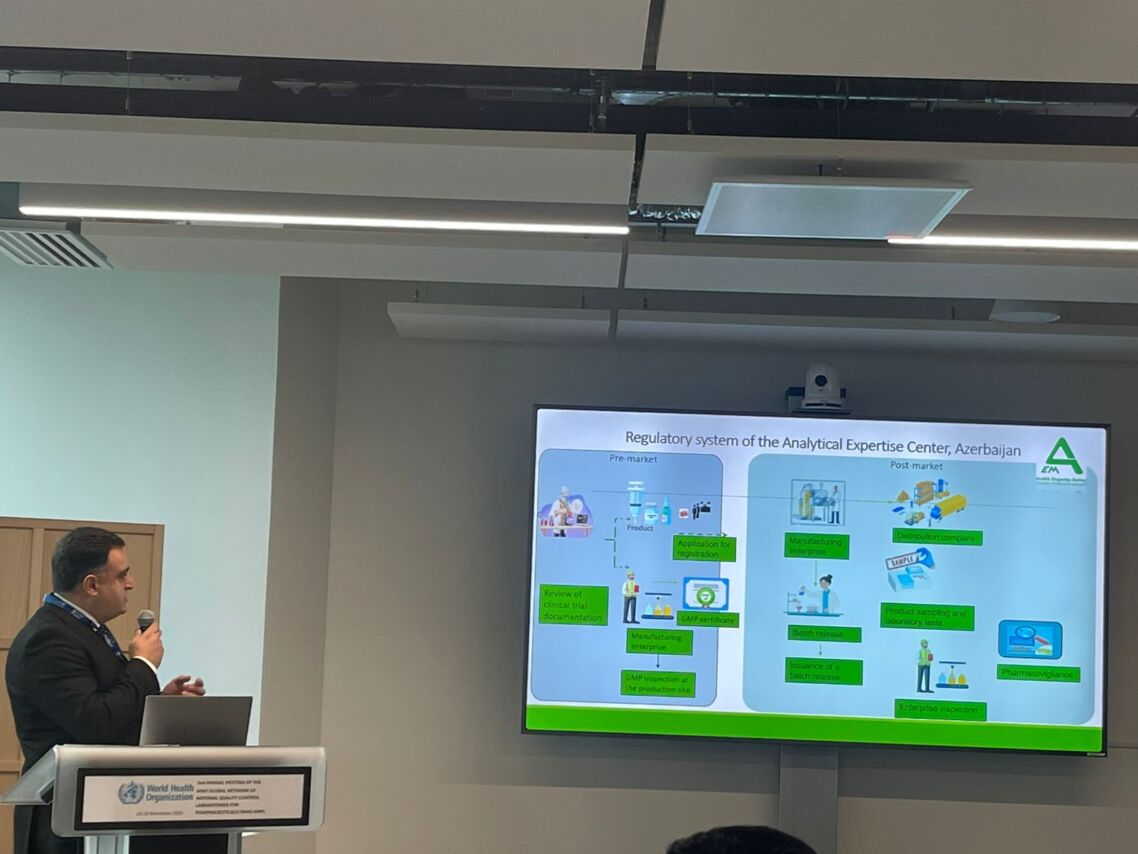
The meeting was attended by Murad Suleymanov, Director General of the Analytical Expertise Center of the Ministry of Health of the Republic of Azerbaijan, which serves as the national regulatory authority in this field, and by Chingiz Shukurov, Head of the Laboratory for the Expertise and Quality Control of Medicinal Products at the Center. During the meeting, Laboratory Head Chingiz Shukurov delivered a presentation on the activities of the Analytical Expertise Center and on the control of the quality of medicinal products in Azerbaijan.
Representatives of the member countries of the aforementioned networks also presented reports on their experiences in this field. During the meeting, WHO experts delivered presentations on new approaches and reliable recognition practices in the field of medicinal product quality control worldwide.
Reports on the identification of impurities in medicinal products, effective and modern methods to combat substandard and falsified medicines, WHO prequalified laboratories, and existing procedures in this field were also met with great interest. In particular, presentations on the use of in vitro testing instead of animal testing for the release of biopharmaceutical product batches, on the relevant monographs in the European Pharmacopoeia, as well as on many new trends in the field, attracted the attention of the participants.